About
Coordination and Access for European COVID-19 Adaptive Platform Trials
The importance of cross-border cooperation and coordination in the fight against the global COVID-19 pandemic has been highlighted since it began. In particular, international clinical research efforts need to be connected and synchronized to ensure that results are obtained rapidly while remaining robust, reproducible and reliable.
With this aim in mind, initially, two ambitious projects financed by the European Commission, EU-RESPONSE and RECOVER, established a joint coordination module between them. This module allows them to address the most pressing research questions through large adaptive platform trials (APT), while avoiding duplications and maximizing the use of resources. This collaboration was further extended to incorporate ECRAID-Prime and VACCELERATE into the module. As new COVID-19 APT projects funded by the European Commission have arisen, they have integrated this joint coordination module. The scope of which has also expanded to include new outbreaks including MPox.
The EU-funded projects
 The RECOVER project has a multidisciplinary approach to tackle the COVID-19 pandemic. It provides support for the activities and the expansion of the REMAP-CAP APT in Europe. Furthermore, the RECOVER project incorporates observational studies in the primary care and hospital settings, clinical biological studies including transcontinental cooperation, epidemiologic and modeling studies, and social science studies.
The RECOVER project has a multidisciplinary approach to tackle the COVID-19 pandemic. It provides support for the activities and the expansion of the REMAP-CAP APT in Europe. Furthermore, the RECOVER project incorporates observational studies in the primary care and hospital settings, clinical biological studies including transcontinental cooperation, epidemiologic and modeling studies, and social science studies.
 EU-RESPONSE is a 5-year project that includes two broad Pan-European APTs, DisCoVeRy and EU-SolidAct. The EU-RESPONSE consortium is composed of 22 partners, bringing together multidisciplinary partners from across the continent.
EU-RESPONSE is a 5-year project that includes two broad Pan-European APTs, DisCoVeRy and EU-SolidAct. The EU-RESPONSE consortium is composed of 22 partners, bringing together multidisciplinary partners from across the continent.
 ECRAID-Prime is an ambitious project to pioneer Europe’s first adaptive platform trial on COVID-19 therapeutics in the primary care setting. Its goal is to establish safety and efficacy of COVID-19 and COVID-like-illness treatments within the community to help speed up recovery and/or to prevent deterioration of illness and hospitalization.
ECRAID-Prime is an ambitious project to pioneer Europe’s first adaptive platform trial on COVID-19 therapeutics in the primary care setting. Its goal is to establish safety and efficacy of COVID-19 and COVID-like-illness treatments within the community to help speed up recovery and/or to prevent deterioration of illness and hospitalization.
 VACCELERATE is a European clinical research network for the coordination, conduct and acceleration of (COVID-19) vaccine trials in Europe. It brings together different stakeholders involved in vaccine development across the continent and has developed a platform for trial design and conduct. To speed up the start of trials, VACCELERATE offers consultation to vaccine developers on study design, a Site Network of capable trial sites and a European Volunteer Registry for people interested in participating in clinical studies.
VACCELERATE is a European clinical research network for the coordination, conduct and acceleration of (COVID-19) vaccine trials in Europe. It brings together different stakeholders involved in vaccine development across the continent and has developed a platform for trial design and conduct. To speed up the start of trials, VACCELERATE offers consultation to vaccine developers on study design, a Site Network of capable trial sites and a European Volunteer Registry for people interested in participating in clinical studies.
The coordination module
The shared coordination module ensures optimal collaboration and complementarity between trials in the EU and abroad. It is composed of the Trial Coordination Board (TCB), the Joint Access Advisory Mechanism (JAAM) and the Adaptive Platform Trial Toolbox.
Co-led by the European Clinical Research Infrastructure Network (ECRIN) and the Norwegian Institute of Public Health (NIPH), the coordination module strives to bring to the table all key stakeholders for platform trial development and implementation, to discuss and make recommendations on the development of the European platform trials, and to identify and develop strategic partnerships. Through the JAAM it provides a single access point for new and repurposed drugs, developed by the industry or academia, to join the European APT. Furthermore, this module provides an array of tools that can be used to design, implement and manage APT for COVID-19 and other disease areas.
The adaptive platform trials accessed through the JAAM
REMAP-CAP stands for Randomised, Embedded, Multi-factorial, Adaptive Platform trial for Community-Acquired Pneumonia. This global adaptive platform trial, aimed towards finding the best treatement for community-acquired pneumonia, is multifactorial and flexible, allowing it to rapidly adapt as planned, to include COVID-19 patients. Supported by the RECOVER project, REMAP-CAP continues to investigate several treatments for COVID-19 and supports a growing network of study sites across the European continent.
The design of the REMAP-CAP for participants with suspected or proven COVID-19 is as follows:
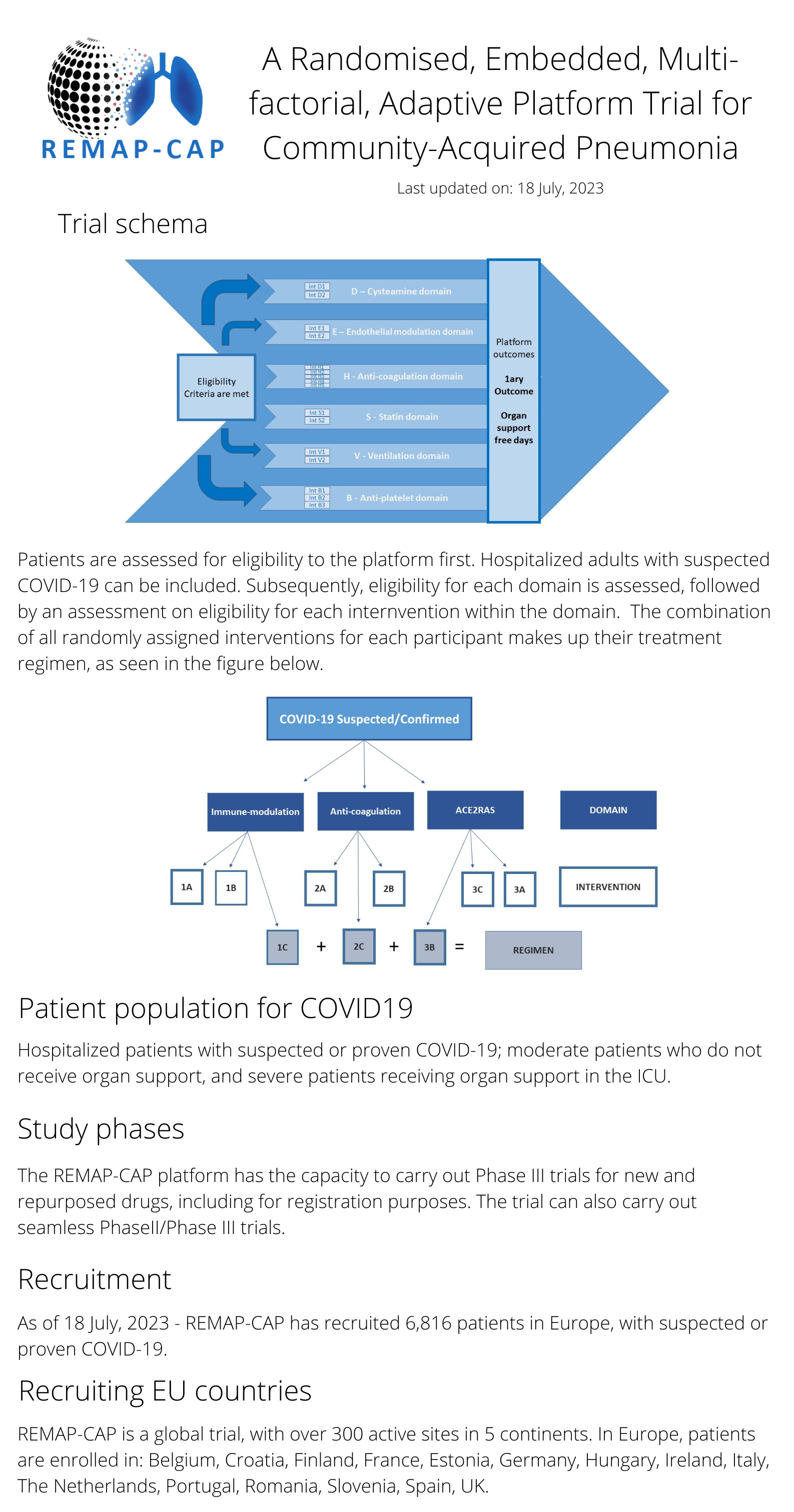
To learn more on how to become involved REMAP-CAP trial click here. Additional details on the trial can be found here.
The EU-SolidAct platform kicked off in 2021 and will include study sites in 15 countries. Developed with the EU-RESPONSE project, this platform has the capacity to implement Phase II and Phase III trials, and can evolve to include new study arms for hospitalized patients with moderate or severe COVID-19.
The following infographic summarizes the design of the EU-SolidAct platform:
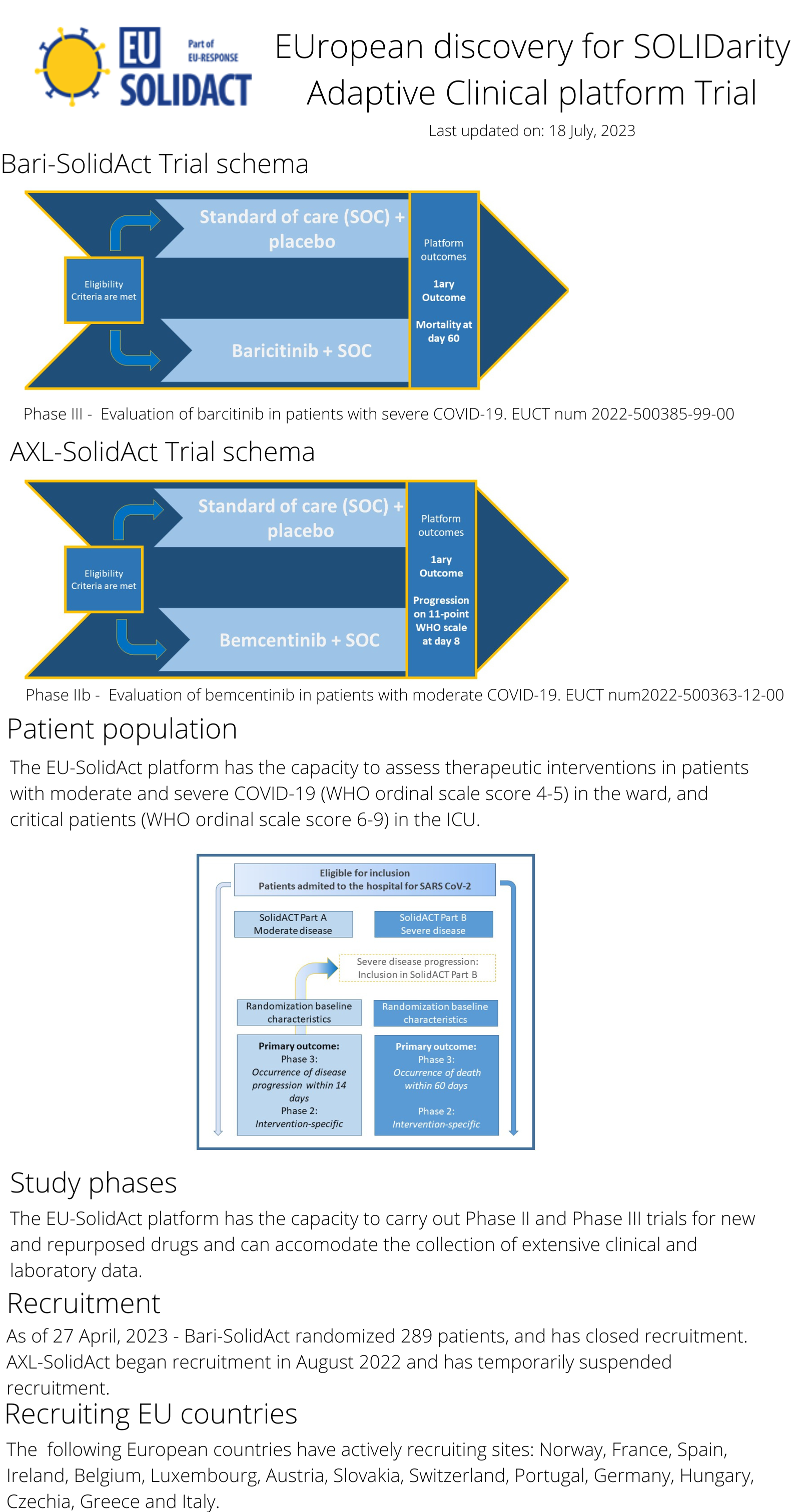
To learn more about how to propose a new study arm in the EU-SolidAct trial click here.
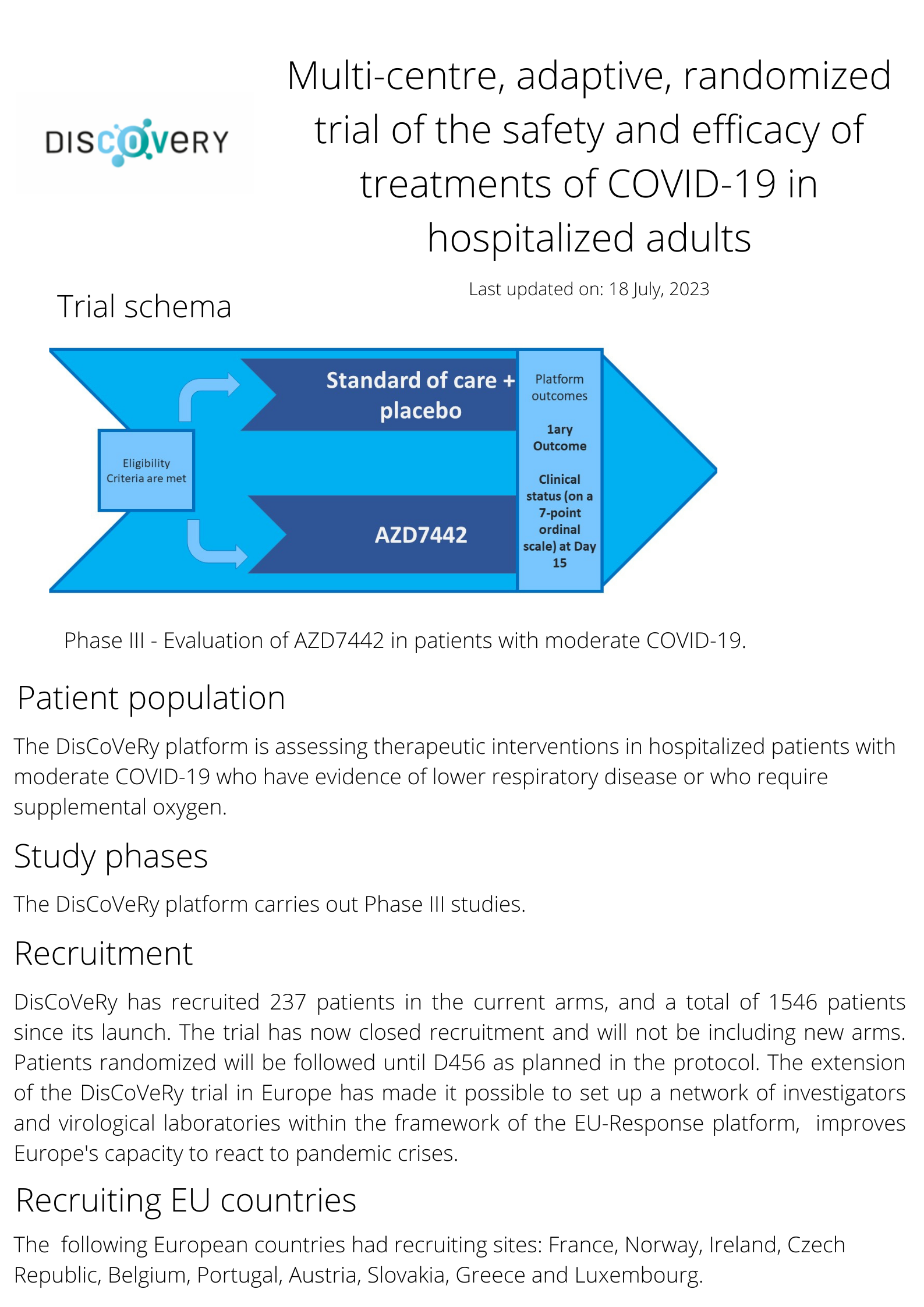
The DisCoVeRy trial was developed as an “add on” trial to the WHO Solidarity trial and initiated in France. Within the framework of the EU-RESPONSE project, DisCoVeRy was able to expand geographically, with the ambition of including 13 countries across Europe, and has evolved to include new study arms. The trial has assessed hydroxichloroquine, lopinavir/ritonavir with and without interferon β-1a and remdesivir.
The trial is currently as follows:

ECRAID-Prime is a first-of-its-kind European Adaptive Platform Trial in the primary care setting. This randomized, double-blind, multi-country, multi-center platform trial assesses therapeutics for COVID-19 and COVID-like-illness and has the capacity to perform Phase I, Phase II and Phase III evaluations. The platform is open to including new interventions that are adapted for non-hospitalized patients in the community and primary care settings.
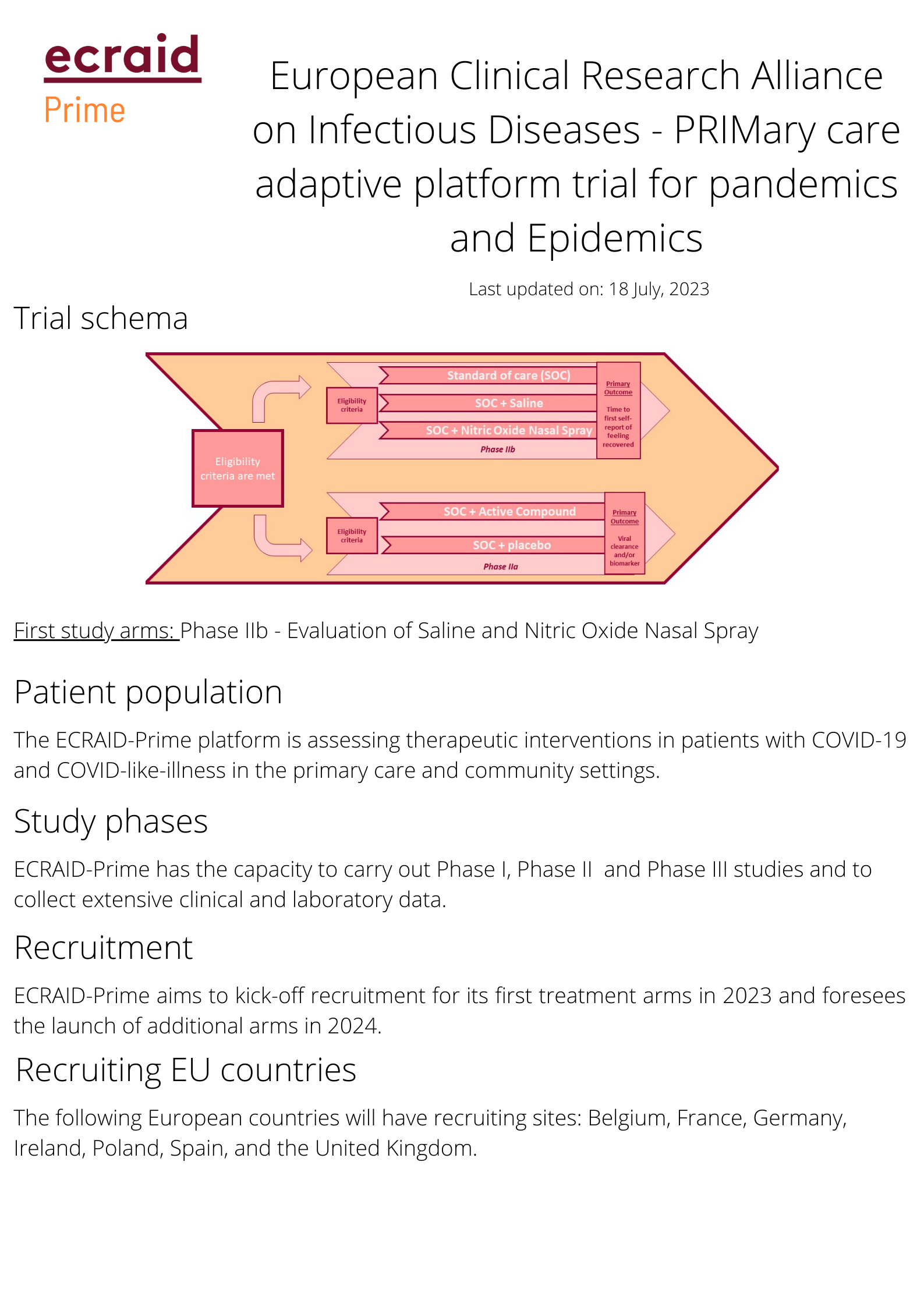 To learn more about how to propose a new study arm in the ECRAID-Prime trial click here.
To learn more about how to propose a new study arm in the ECRAID-Prime trial click here.