JAAM
The Joint Access Advisory Mechanism
- JAAM activities were discontinued at the end of December 2024 -
The Joint Access Advisory Mechanism (JAAM) is the entryway for the therapeutic European COVID-19 adaptive platform trials (APTs). It has recently expanded to include other respiratory syndromes. It is the single body, common to the EU-SolidAct, REMAP-CAP and ECRAID-Prime trials, that assesses requests from investigators or industry looking to test their compound in one or more of these trials.
Composition
The JAAM is composed of a panel of seven renowned experts that are fully independent from the EU-funded APTs. They cover the following fields of expertise:
- Pre-clinical research, animal models and basic virology
- Clinical virology and infectious diseases
- Immunology and inflammation
- Pharmacology
- Trial methodology
- Health technology assessment
The coordinators of the EU RESPONSE and RECOVER projects, and the principal investigators of the EU-SolidAct, REMAP-CAP in Europe and ECRAID-Prime trials are observers of the JAAM.
How it works
The JAAM meets regularly, to assess requests for access according to the following criteria :
1- Public health interest;
2- Scientific, medical and ethical soundness;
3- Appropriate patient population, comparator and outcomes;
4- Promotion of coordination and optimal use of resources.
Download the JAAM brochure
Process and timelines
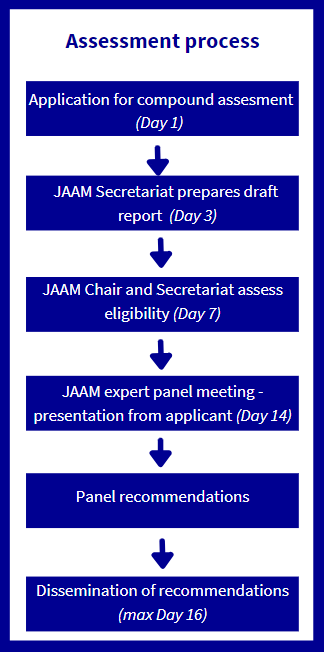
The applicant must first submit their proposal directly to the JAAM Secretariat at covid19trialseu@ecrin.org. Applications are intended to be prepared for a phase II trial with supporting documents from the earlier work. The secretariat is available to discuss the best timing for submission. Early contact with the secretariat is welcomed if you have a clear plan for drug development and you are willing to receive follow up emails. Applicants will be requested to cover all key characteristics of the compound and use the checklist to ensure all necessary elements are included in their submission. The JAAM Secretariat will then ensure all information provided by the applicant is complete (and may request additional information), and will consult the JAAM chair for eligibility of the compound.
Assessment process of the JAAM: